Chemistries
Overview
Waterville Analytical instrumentation uses flow injection analysis, FIA, to deliver a sample to a flow cell where analyte-specific chemiluminescence occurs. Analyte specificity is achieved by careful selection of the chemiluminescent reagent and analytical conditions such as: reaction pH, reaction time, and masking reagents.
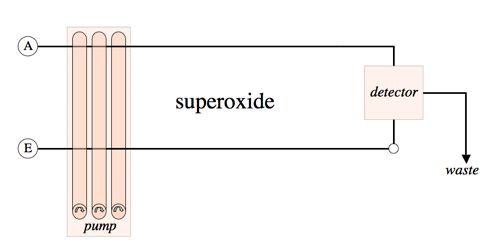
The diagram below shows the general fluidics configuration of the Waterville Analytical instrument.
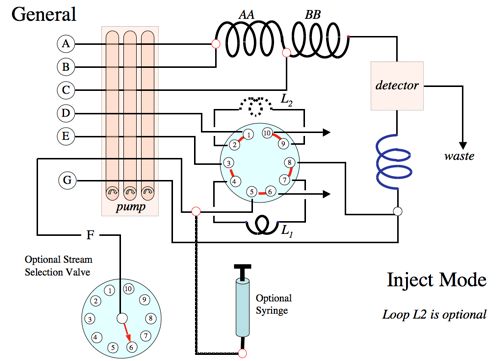
The table below shows reagent recipes for specific analytes. You may click on the rows for specific analytes to obtain more information.
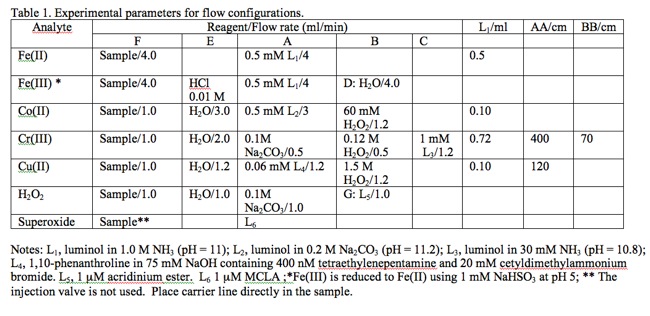
Fe(II)
Ferrous Iron Analysis in seawater, freshwater, and boiler water. Ferrous iron is oxidized by dissolved molecular oxygen to produce superoxide, and subsequently the peroxycarbonate radical. These species oxidize luminol in two steps to produce chemiluminescence.
mechanism_a.gif)
mechanism_b.gif)
Implementation:
 diagram.jpg)
References
Emmenegger, L., D. W. King, et al. (1998). "Oxidation Kinetics of Fe(II) in a Eutrophic Swiss Lake." Environmental Science and Technology 32(19): 2990-2996.
King, D. W. (1998). "Role of Carbonate Speciation on the Oxidation Rate of Fe(II) in Aquatic Systems." Environmental Science and Technology 32(19): 2997-3003.
King, D. W., H. A. Lounsbury, et al. (1995). "Rates and Mechanism of Fe(II) Oxidation at Nanomolar Total Iron Concentrations." Environmental Science and Technology 29(3): 818-24.
Powell, R. T., D. W. King, et al. (1995). "Iron distributions in surface waters of the south Atlantic." Marine Chemistry 50(1-4): 13-20.
Roy, E. G., C. Jiang, M. et al. (2008). "Determining Subnanomolar Iron Concentrations in Oceanic Seawater Using a Siderophore-Modified Film Analyzed by Infrared Spectroscopy" Analytical Chemistry 80(12) 4689-4695.
Xiao, C., D. W. King, et al. (2000). "Study of enhancement effects in the chemiluminescence method for Cr(III) in the ng/l range." Analytica Chimica Acta 415: 209-219.
Xiao, C., D. A. Palmer, et al. (2002). "Carbon Dioxide Effects on Luminol and 1,10-Phenanthroline Chemiluminescence." Analytical Chemistry 74(9): 2210-2216.
Fe(III)
Ferric iron is reduced to ferrous iron using sodium bisulfate.
mechanism.gif)
Samples should be degassed of oxygen before analysis for best analytical performance. Ferrous iron is detected using the same method as listed for Fe(II).
References
Roy, E. G., C. Jiang, M. et al. (2008). "Determining Subnanomolar Iron Concentrations in Oceanic Seawater Using a Siderophore-Modified Film Analyzed by Infrared Spectroscopy" Analytical Chemistry 80(12) 4689-4695.
Hydrogen Peroxide
Hydrogen peroxide analysis in seawater, freshwater, and biological fluids. An acridinium ester-based CL reagent (AE-CL), 10-methyl-9-(p-formylphenyl)-acridinium carboxylate trifluoromethanesulfonate, is used for the analysis of hydrogen peroxide in seawater (Cooper et al. 2000, King et al. 2007).
The proposed AE-CL mechanism is shown below. In this system, the hydrogen peroxide anion initially attacks the acridine moiety. Under alkaline conditions, the ROO - adduct undergoes intermolecular rearrangement forming the dioxetane intermediate. The intermediate rapidly decomposes (ca. ms) to the reaction products including the electronically excited intermediate N-methylacridone, which chemiluminesces at approximately 470 nm.
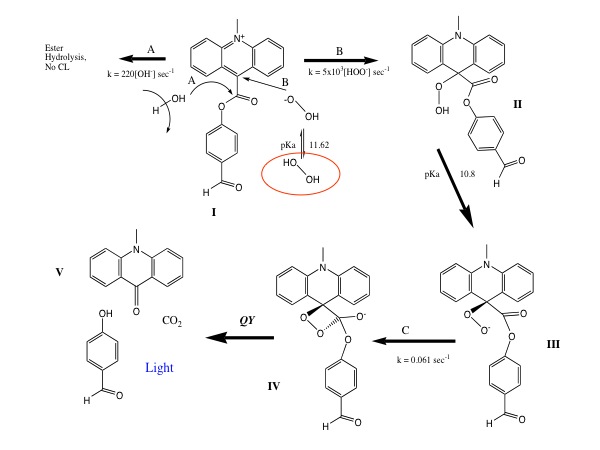
Implementation:
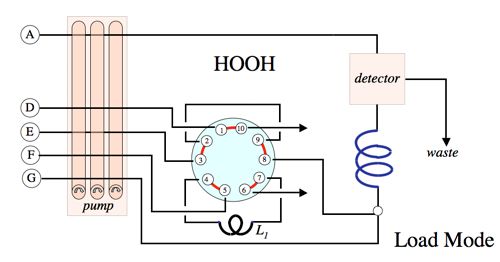
References
King, D, W Cooper, S Rusak, B Peake, and J Kiddle. (Jan 2007) "Flow Injection Analysis of H2O2 in Natural Waters Using Acridinium Ester Chemiluminescence: Method." Analytical Chemistry.
Superoxide
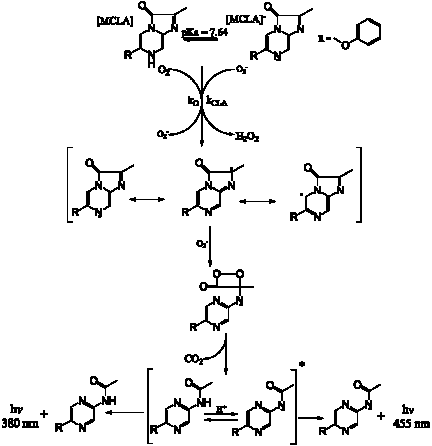
Superoxide is detected using the chemiluminescence reagent MCLA
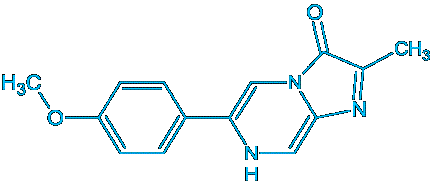
Chemiluminescent reagent MCLA [2-methyl-6-(4-methoxyphenyl)-3,7-dihydroimidazo[1,2-a]pyrazin-3-one, HCl]
MCLA in a well-buffered solution at pH 6 is mixed with seawater or freshwater samples directly in front of the PMT. Typical detection limits are 50 pM.
Implementation:
References
Zheng, J; S R Springston, Weinstein-Lloyd. (2003). Analytical Chemistry 75, 4696-4700
Akutsu, K., H Nakajima, T Katoh, S. Kino, K J Fujimori. (1995). Chem. Soc. Perkin Trans. 2, 8, 1699-706
Fujimori, K, H Nakajima, K Akutsu, M Mitani, H Sawada, M J Nakayama. (1993). Chem. Soc. Perkin Trans. 2, 12, 2405-9
Rose, A. L, J W Moffett, T D Waite. (2008). "Determination of Superoxide in Seawater Using 2-Methyl-6-(4-methoxyphenyl)-3,7- dihydroimidazo[1,2-a]pyrazin-3(7H)-one Chemiluminescence." Analytical Chemistry 80 (4), 1215-1227
